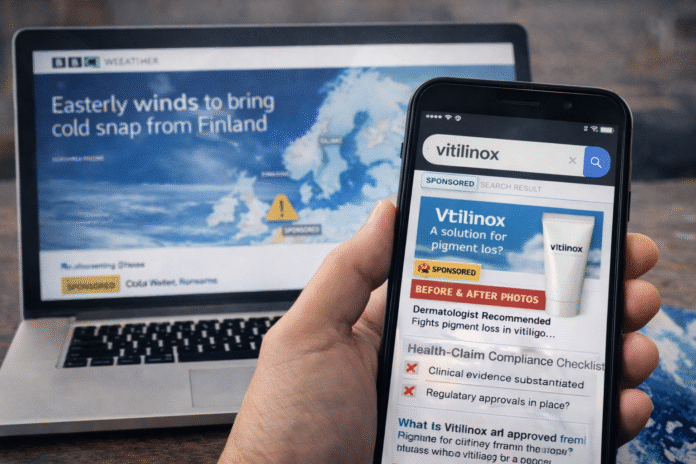
A winter cold snap can change what people buy, what they search for, and how much they spend on everyday essentials. In the UK, the Met Office has long noted how easterly winds linked to high pressure over Scandinavia can drag in bitterly cold air conditions often referred to as the “Beast from the East.” In plain terms, a wind blowing from Finland or at least from the same broad direction across northern Europe can make Britain feel colder, drier, and harsher on skin.
This year, that seasonal pattern is colliding with a different force: the online marketplace for health and skincare products, where advertising is often triggered by search demand. One term increasingly popping up across ads and web pages is vitilinox, a name that appears to be used in connection with pigmentation and vitiligo-related marketing, even as authoritative public information about the product itself remains limited and inconsistent.
For consumer-facing platforms, advertisers, and payment firms, the financial story is familiar. When demand rises quickly whether due to cold weather, a viral keyword, or heightened anxiety about appearance marketing accelerates. The risk is that the fastest-growing campaigns are not always the most careful, especially when they tip into health claims that require strong evidence and, in some cases, regulatory authorisation.
That is becoming more visible in US and UK enforcement trends. In the United States, the Federal Trade Commission’s health products compliance guidance says claims about benefits and safety must be truthful, not misleading, and supported by science. In Britain, the Advertising Standards Authority warns that if the Medicines and Healthcare products Regulatory Agency classifies a product as a medicine, marketers generally need the appropriate authorisation before promoting it as such. The MHRA has also published guidance explaining how product “intended use” and claims can push an item across regulatory lines, including the boundary between cosmetics and medicines.
Vitilinox, vitiligo, and the gap between marketing and verification
Vitiligo is a condition in which skin loses pigment, leading to lighter patches. Medical authorities describe multiple treatment approaches, including topical medicines and light-based therapies, and they emphasize that results can vary and take time. In the US, the American Academy of Dermatology notes that ruxolitinib cream is an FDA-approved option for certain patients with non-segmental vitiligo, while also describing other treatments that may be used under medical supervision.
Against that medical backdrop, “vitilinox” is showing up as a brand-like label online. The most concrete, checkable footprint is that “VITILINOX” appears in a Torrent Pharmaceuticals scheme document as a trademark entry, listed under trademark classes commonly associated with cosmetics and medicinal products (classes 3 and 5), alongside application numbers. That does not, by itself, establish what the product is, how it is formulated, or whether any advertised claims are backed by clinical evidence. It simply indicates that the name has appeared in trademark-related paperwork.
Outside of official or authoritative medical sources, many pages describing vitilinox are promotional or blog-style and do not provide verifiable clinical data. That matters because in health-related categories, the distinction between “cosmetic” positioning and “treatment” positioning is not just semantic it can change which rules apply.
In the US, the Food and Drug Administration explains that whether something is regulated as a cosmetic or a drug depends on intended use, which is largely inferred from claims. If a product claims to treat or prevent a disease, it is more likely to be regulated as a drug, with different requirements. In the UK, MHRA guidance similarly stresses that claims can determine classification, and that cosmetic claims should emphasize cosmetic purposes such as cleansing or keeping skin in good condition.
That is the financial tension for advertisers. A softer message about “appearance” is easier to run and less likely to trigger regulatory scrutiny. A stronger message implying treatment outcomes may convert better especially when people are anxious but can carry higher enforcement and reputational risk.
Why winter weather can move markets for skincare and “solutions”
Cold spells do not just raise heating bills; they often shift household spending towards products that promise comfort moisturisers, barrier creams, and other skin-care items. When the Met Office describes the UK’s cold, wintry pattern tied to easterly winds from continental Europe, it is also describing a situation that can change consumer behaviour.
In digital advertising, those behaviour shifts become signals. Search queries rise, and ad auctions react in real time. Brands and affiliates bid on keywords, platforms sell placement, and publishers build pages targeting those terms. In health-adjacent categories, that can lead to a surge of claims that range from cautious to sweeping.
The market incentives are straightforward:
Advertising in high-intent health queries can be expensive, but the payoff can also be high because a user who is searching for a specific condition or product name is closer to buying than a casual browser. That drives competition, and competition can encourage “aggressive” copywriting that tests regulatory boundaries.
For platforms, the cold-weather spike can translate into higher ad revenue, but it also increases moderation burdens. Health claims are among the hardest to police at scale because they can be implied rather than stated outright. The FTC’s guidance warns advertisers that both express and implied claims matter and must be supported by adequate evidence.
For payment providers, the risk is downstream. If a product is marketed with strong promises and customers feel misled, disputes rise. Chargebacks and complaints can increase costs, and repeated problems can lead to merchant-account restrictions. In a high-volume environment, those frictions can spread beyond one product to entire categories.
Table
| What consumers may see online | What can be verified from stronger sources | Why it matters for finance and platforms |
|---|---|---|
| “vitilinox” described as a solution for pigment loss | “VITILINOX” appears as a trademark entry in a Torrent Pharma scheme document (classes 3 and 5 listed with application numbers). | Branding can scale quickly; unclear claims can increase refunds, disputes, and compliance pressure |
| Marketing language that sounds like treatment outcomes | Medical authorities describe vitiligo as a condition with varied treatment paths; the AAD notes an FDA-approved ruxolitinib cream option for certain patients, alongside other approaches. | Treatment claims can trigger stricter ad standards, higher legal exposure, and greater platform enforcement |
| “Cosmetic” wording paired with medical-sounding implications | FDA and UK guidance emphasize that intended use and claims can determine whether a product is regulated as a cosmetic or a drug/medicine. | Classification affects what claims can be made and what authorisations may be needed |
The regulatory direction of travel
Regulators are not banning marketing. The message is narrower: if you are making objective health claims, you need evidence, and in some cases you may need authorisation.
In the US, the FTC has said its health-products guidance reflects extensive enforcement history and is meant to help marketers avoid claims that are not supported by appropriate science. In the UK, the ASA’s advice on medicinal claims underscores that if the MHRA classifies something as a medicine, it generally needs a licence and marketing authorisation before it is sold or marketed as such. MHRA guidance also draws lines around cosmetic claims, emphasising cosmetic purposes and warning against medicinal implications that could reclassify a product.
For companies, the operational challenge is that global marketing campaigns often run across borders. A claim that is phrased carefully for one jurisdiction may still be non-compliant in another. This can be particularly tricky for skincare, where the same product can be sold as “beautifying” on one page and “treating” on another, depending on the audience and the ad copy.
That compliance burden can become a cost centre. Larger firms may invest in claim substantiation, legal review, and ad monitoring tools. Smaller sellers may rely on templates, affiliates, or automated ad generation, increasing the odds of overreach.
What to watch next
The near-term market question is whether “vitilinox” remains a niche keyword or becomes a broader consumer brand that attracts mainstream scrutiny. If it scales, the advertising and compliance questions will scale with it, especially in winter periods that amplify skin-related purchasing.
A second watchpoint is platform enforcement. Ad platforms and social networks face growing pressure to stop misleading health claims, and the standards applied to one category often spill into others. The more platforms tighten the rules, the more marketers may shift towards vaguer language claims that “support” or “help” without stating measurable outcomes because it is less likely to be challenged.
Third, weather remains an underappreciated driver of consumer demand. A wind blowing from Finland may be a shorthand phrase, but the economic effect is real: colder air can raise attention to skin comfort and appearance, which can lift demand for products at every price point, from basic moisturisers to premium creams. The line between legitimate demand and opportunistic marketing is where regulators and platforms are now concentrating.
For readers, the practical takeaway is cautious rather than alarmist. Vitiligo is a real medical condition with treatments described by reputable medical organisations, and claims about any product in that space deserve careful scrutiny. For markets, the takeaway is structural: as health-related advertising becomes more search-driven and seasonal, the “cost of trust” rises for platforms, for payment firms, and for advertisers that want to scale without triggering enforcement.
FAQ
Q1: What is vitiligo, in simple terms?
Vitiligo is a condition where skin loses pigment, leading to lighter patches. Medical sources describe multiple treatment approaches and note that responses can vary.
Q2: Is “vitilinox” an approved medical treatment?
I did not find an authoritative medical-source page establishing vitilinox as an approved treatment. The strongest verifiable reference located was a trademark listing in a Torrent Pharma scheme document.
Q3: Why does “wind blowing from Finland” matter in a finance story?
Cold easterly patterns associated with Scandinavia can drive winter conditions in the UK, which can shift consumer spending and online search demand fueling advertising activity in categories like skincare.
Q4: What do regulators expect from health-product advertising?
In the US, the FTC says health benefit claims should be truthful, not misleading, and supported by science. In the UK, the ASA and MHRA guidance highlights that medicinal claims can require authorisation and that product claims can affect classification
Conclusion
The mix of vitilinox marketing and a wind blowing from Finland captures how quickly seasonal demand can amplify health-adjacent claims online. Cold snaps tend to push more people into searching for “solutions,” and search-driven advertising can reward the loudest promises whether or not they are well-supported. With regulators in the US and UK focused on substantiation and classification, the next test will be how brands and platforms balance fast-moving consumer demand with stricter standards for what can be claimed, and how clearly it can be verified.